ACANDIS
Driven by passion, powered by science –
changing lives through medical technology.
Tiny implants that save lives every day.
Acandis is a forerunner and pioneer of current and future innovations in the field of minimally invasive endovascular technologies and solutions for the treatment of neurovascular diseases based on miniaturised medical technology.
We are a dynamic team with high motivation, creativity and initiative, dedicating ourselves to technological and medical challenges.
Technology and innovation drives us - "miniaturisation" is our motto.
We cultivate an open corporate culture with flat hierarchies and rely on the innovative strength of our employees:
"We always give every good idea a chance", says CEO Dr. Andreas Schüßler.
Innovation meets spirit:
That's us.
We are proud to have made it into the TOP 100 of the most innovative SMEs for the third time!
TOP 100 is the only independent award for innovation management in Germany. Our company was evaluated on the basis of the categories "Innovation-promoting top management", "Climate of innovation", "Innovative processes and organization", "External orientation / open innovation" and "Innovation success".
The TOP 100 seal underlines the innovative strength and therefore the future viability of our company.





About us
Acandis is a highly innovative, fast-growing, owner-managed medical technology company.
Since its founding in 2006, Acandis, based in Pforzheim, Germany,
has specialised in the development, manufacturing and marketing of innovative products for the treatment of neurovascular diseases
- high-tech medical devices for acute and preventive stroke treatment.
In close exchange with physicians and hospitals, the focus is on optimal clinical support as well as their continuous further development - for the benefit of the patients. Acandis strives to grow steadily and operate internationally in order to make our innovations accessible to more people
around the world.
Highlights of our History.
2013 Market Launch
- NeuroSlider® DLC Microcatheter
- DERIVO® Embolisation Device
- NeuroBridge® Catheter
- APERIO® Thrombectomy Device
2014 Market Launch
- ACCLINO® flex Stent
- APERIO® Thrombectomy Device – Range Extension
- NeuroSpeed® PTA Balloon Catheter – Range Extension
- DERIVO® Embolisation Device – Range Extension
- ACCLINO® flex Stent – Range Extension
2015 Market Launch
- CREDO® Stent
- NeuroSpeed® PTA Balloon Catheter – Range Extension
- DERIVO® Embolisation Device – New Visibility Concept
- ACCERO® Stent
2016 Market Launch
-
- CREDO® Stent First-In-Man
- NeuroBridge® Catheter – Range Extension
- ACCLINO® flex plus Stent
2018 Market Launch
- NeuroSlider® 27 Microcatheter (DLC)
- CREDO® Stent – New Generation
- NeuroSpeed® PTA Balloon Catheter – Optimisation
- ACCERO® Stent – Optimisation
- DERIVO® mini Embolisation Device
- ACCLINO® flex Stent – Range Extension
- ACCLINO® flex plus Stent – Range Extension
2019 Market Launch
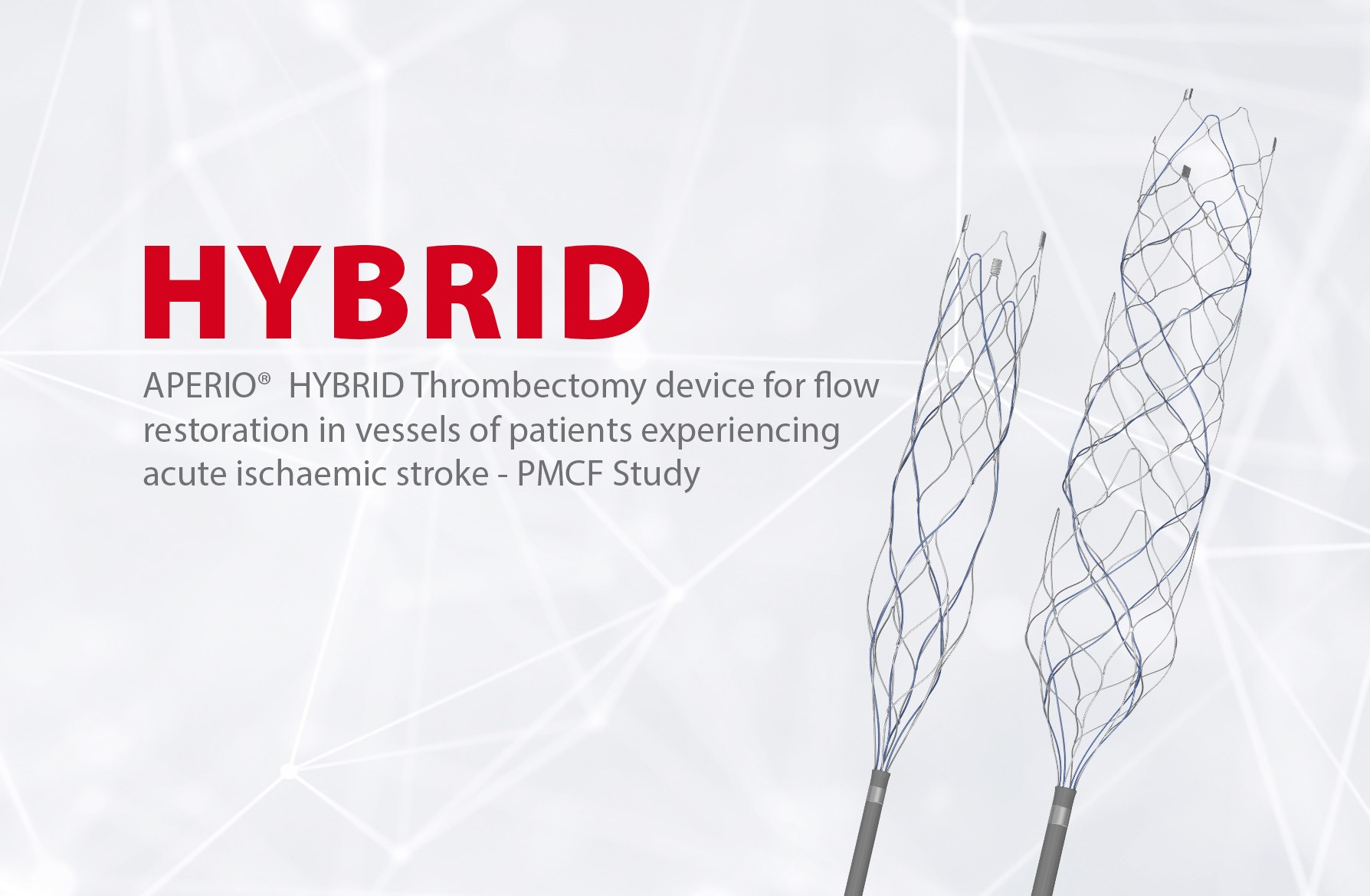
- APERIO® Hybrid Thrombectomy Device
- DERIVO® mini Embolisation Device – Optimisation
- NeuroSlider® Microcatheter DLC
2020 Market Launch
- APERIO® Hybrid17 Thrombectomy Device
- ACCLINO® flex plus Stent – Range Extension
- CREDO® Stent – Range Extension
- DERIVO® 2 Embolisation Device
- ACCERO® Rex Stent – Range Extension
- NeuroBridge® A70 Catheter
2021 Market Launch
- CREDO® Stent – Acute Indication
- CREDO® Stent – RE
- APERIO® Hybrid21 Thrombectomy Device
- NeuroSlider® 27/27 pro/39 Microcatheter DLC – RE
- ACCERO® Stent – Optimisation
- CARESTO® flex Stent
- CARESTO® heal Stent
- DERIVO® heal Embolisation Device
- DERIVO® 2heal® Embolisation Device
- CREDO® heal Stent
- ACCERO® heal Stent
- ACCLINO® heal Stent
- DERIVO® peripher – Embolisation Device
- CREDO® Stent – RE